Health software development and regulatory consulting
We are a medical device consultancy. We provide expertise on software development for medical devices.
Health software development and regulatory consulting
We are a medical device consultancy. We provide expertise on software development for medical devices.
ISO 13485
IEC 62304
EU MDR
FDA CFR
About Sunbird Medical Devices
We provide trainings, consulting and fully compliant software development for medical devices.

Lower the learning curve on doing medical device development (more speed means quicker to market)

Lower the learning curve on doing medical device development (more speed means quicker to market)

Increase efficiency of your software engineering team and accelerate your market submission

Increase efficiency of your software engineering team and accelerate your market submission

TRUSTED BY
















What we offer
We offer consulting services for software medical device software.
Our services include comprehensive trainings and workshops and consulting services, as well as software development and UX, setting us apart from the competition.

Training and Workshops
Our team of experienced consultants routinely train customers in subjects like IEC 62304, cybersecurity for medical devices, and usability engineering for medical devices
WORKSHOPS FOR:
- Health tech startups
- Biotech companies
- Software engineers
- Regulatory consultants

Consulting
Sunbird Medical Devices helps clients with all aspects of software development, including documentation, setting up a development team, increasing compliance, and optimizing your team’s velocity within the rules.
SPECIALISED FOR:
- Project managers
- Health tech startups
- Product Owners
- Software engineers

Software Development and UX
We provide software development as a service including documentation writing and seamless integration into your eQMS. We offer development, testing (verification) and DevOps services.
FOR:
- Health tech startups
- Software teams
- Project managers

Training and Workshops

Consulting
Sunbird Medical Devices helps clients with all aspects of software development, including documentation, setting up a development team, increasing compliance, and optimizing your team’s velocity within the rules.

Software Development and UX
WORKSHOPS FOR:
- Health tech startups
- Biotech companies
- Software engineers
- Regulatory consultants
SPECIALISED FOR:
- Project managers
- Health tech startups
- Product Owners
- Software engineers
FOR:
- Health tech startups
- Software teams
- Project managers
Our approach

1. Intake Quickscan
Schedule a call with our team for getting an overview of the your specific situation, and how our offer can help you as efficient as possible. This can be a combination of services, from workshops to hands-on consulting, as well as being a partner in the software dev and ux side.
2. Customized offer
Our customised offer, combining compliance consulting, training and workshops, and software development and UX support, provides a comprehensive solution for medical device software looking to navigate the complex regulatory environment and ensure that their products meet the highest standards of safety and effectiveness.
3. Execution of services
We follow up on the process by maintaining open communication with our clients, providing regular progress updates, and ensuring that our services continue to meet their evolving needs and requirements.
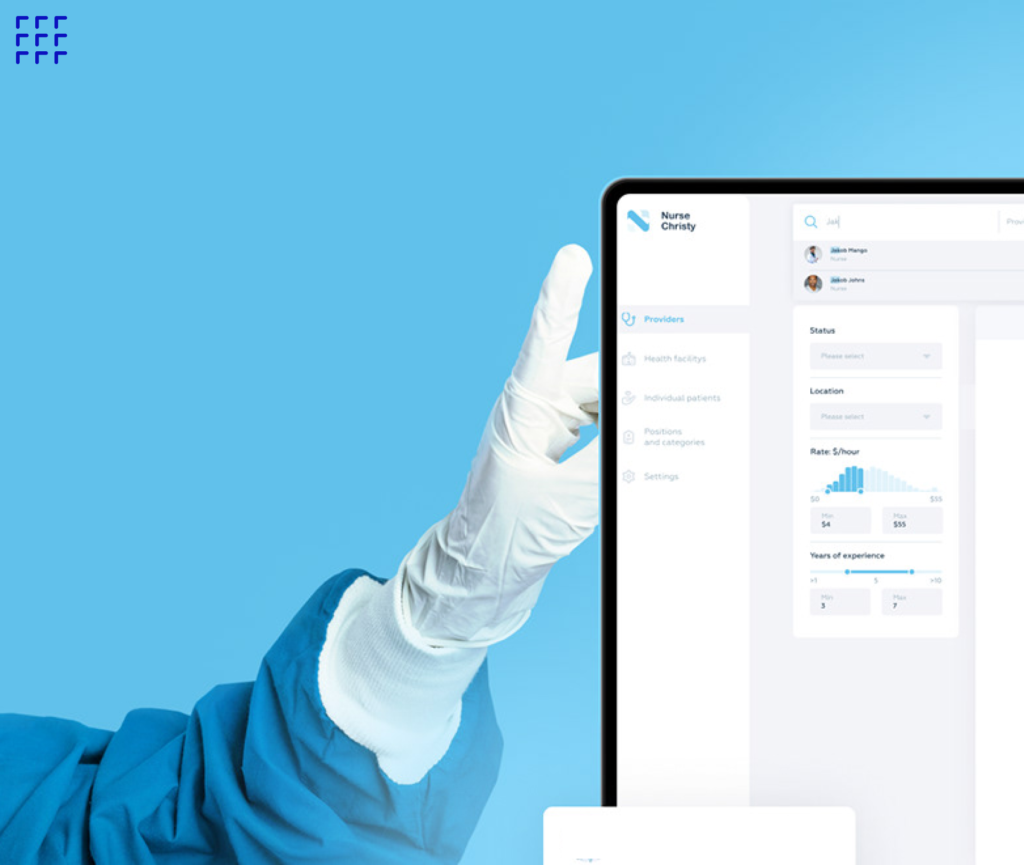
Ready to go next level?
- Get up to speed quickly with required documentation
- Accelerate your market submission
- Increase efficiency of your software engineering team
Ready to go next level?
- Get up to speed quickly with required documentation
- Accelerate your market submission
- Increase efficiency of your software engineering team

Explore our latest
Reliability in medical devices
When we are dealing with medical devices, we expect them to be reliable. But what is reliability in these devices? Reliability is not trust When we say we trust a

The how and the what of external software components (SOUP or OTS software) in medical devices
Currently, when developing software for medical devices, there are two sets of rules that deal with external software components: IEC 62304 and the FDA guidance from 2019 (‘Off-The-Shelf Software Use

The pros and cons of current regulations on using external software components in medical device software (SOUP or OTS)
The regulations around external software components are mostly risk-averse and safety oriented. This negates the benefit of updating components frequently. In this article, we look into this contradiction. Currently, when

Navigating the Waters of Risk Management for Medical Devices
Introduction In the realm of healthcare, patient safety is paramount, and this holds especially true for medical devices. The development, manufacturing, and distribution of medical devices require rigorous risk management

